New Insights in SENP5 Protease Structure May Transform Therapeutics
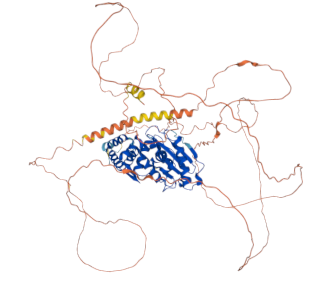
In a groundbreaking study published in *Nature Communications*, researchers from the Institute of Biotechnology and Biomedicine of the Universitat Autònoma de Barcelona (IBB-UAB) have successfully deciphered the three-dimensional structure of the human protease SENP5. This enzyme plays a crucial role in several diseases, including neurodegenerative disorders, cardiovascular diseases, and various cancers. The findings, led by Dr. David Reverter, coordinator of the research and a researcher at UAB, offer promising new therapeutic avenues with potentially minimal side effects.
SENP5 is integral to the post-translational modification process known as SUMOylation, where the Small Ubiquitin-like MOdifier (SUMO) protein modifies other proteins, thereby influencing their functions and regulating numerous cellular activities. Dr. Reverter emphasized the importance of understanding SENP5's structure, stating, "The results we have obtained could facilitate the development of highly specific inhibitors aimed at individual members of the protein family studied, offering new therapeutic opportunities with minimal side effects."
The study reveals a positively charged region within SENP5 that preferentially interacts with SUMO2, a specific isoform of the SUMO protein. This specificity is critical, as it can lead to the development of targeted therapies that inhibit unwanted SUMOylation processes linked to disease. Researchers believe that understanding these mechanisms can provide insights into the complex interactions that underpin various health issues.
The collaboration involved international partners, including Professor Monique Mulder from the University of Leiden in the Netherlands and Virginia Amador from IDIBAPS in Barcelona, highlighting the importance of global scientific collaboration in tackling health challenges.
While the findings present new potential for targeted therapies, they also raise questions about the broader implications for drug development. According to Dr. Sarah Johnson, an associate professor of pharmacology at Stanford University, "Targeting specific proteases like SENP5 could significantly reduce off-target effects seen with conventional therapies, but it requires a deep understanding of each protease's role in various biological contexts."
The implications of this research extend beyond cancer into the realms of neurodegenerative diseases such as Alzheimer’s and cardiovascular health. Dr. Emily Chen, a neurologist at Johns Hopkins University, noted, "Proteases like SENP5 are increasingly recognized as critical players in neurodegeneration. Understanding how they function can lead to innovative approaches in treatment."
The study builds on previous research that highlights the role of SUMOylation in cellular processes. For instance, a 2021 study published in the *Journal of Molecular Biology* outlined how SUMO proteins are involved in gene regulation, which is pivotal in cancer biology. This historical context underlines the importance of ongoing research into proteases and their modifications.
Looking ahead, the findings suggest a pathway for developing drugs that specifically inhibit SENP5, which could lead to more effective treatment options for patients suffering from diseases linked to its dysfunction. The feasibility of such targeted therapies remains an area of active investigation. As Dr. Reverter concluded, "Our work lays the groundwork for future research that could ultimately lead to new, refined therapies for complex diseases."
In summary, the elucidation of SENP5's structure marks a significant advancement in biomedical research, with the potential to transform therapeutic strategies for several debilitating diseases. As researchers continue to explore this protease’s role in health and disease, the hope for tailored, effective treatments becomes increasingly attainable.
Advertisement
Tags
Advertisement





