Structural Insights into Human Protease SENP5: Implications for Disease Treatment
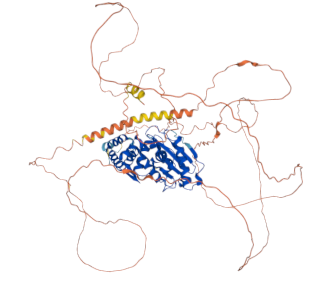
In a significant advancement for biomedical research, a team from the Institute of Biotechnology and Biomedicine of the Autonomous University of Barcelona (IBB-UAB) has successfully elucidated the three-dimensional structure of SENP5, a human protease implicated in various diseases including cancer and neurodegenerative disorders. The findings, published in the journal Nature Communications on June 26, 2025, provide critical insights into the role of post-translational modifications, particularly SUMOylation, in cellular regulation and disease pathogenesis.
Post-translational modifications are essential alterations that proteins undergo after synthesis, influencing their biological activity and physiological processes. Among these modifications, SUMOylation—where Small Ubiquitin-like MOdifier (SUMO) proteins bind to target proteins—plays a crucial role. According to Dr. David Reverter, a researcher at IBB-UAB and coordinator of the study, "The human SENP family plays a fundamental role in the regulation of a variety of cell processes, and its deregulation is involved in many diseases."
The research team employed advanced structural characterization techniques to analyze SENP5 in complex with different SUMO isoforms. The study revealed a positively charged region on SENP5 that directly contributes to its specificity for SUMO2. This specificity is vital as it enables the protease to selectively process SUMO modifications, which can have far-reaching implications for cellular functions and disease mechanisms.
The structural insights gained from this research not only enhance our understanding of SENP5's function but also pave the way for the design of specific inhibitors aimed at targeting this protease. Such inhibitors could offer new therapeutic avenues with reduced side effects, a crucial consideration in the treatment of diseases linked to SENP5 dysregulation.
The potential applications of these findings are substantial. As Dr. Reverter states, "The results we have obtained could facilitate the development of highly specific inhibitors aimed at individual members of the protein family studied, offering new therapeutic opportunities with minimal side effects." This perspective aligns with recent trends in precision medicine, which seeks to tailor treatments based on individual molecular profiles.
Moreover, the implications of this research extend beyond cancer and neurodegenerative diseases. As highlighted in a report from the World Health Organization (WHO) in 2024, the understanding of post-translational modifications is becoming increasingly recognized as a crucial factor in the development and progression of various pathologies, including cardiovascular diseases.
In conclusion, the structural characterization of SENP5 provides a pivotal step towards understanding the intricate mechanisms of SUMOylation and its impact on human health. As further research unfolds, the potential for developing targeted therapies based on these findings could significantly alter the landscape of treatment for diseases associated with this vital protease. Continued collaboration between academic institutions, industry leaders, and healthcare organizations will be essential to translate these insights into clinical applications, ultimately improving patient outcomes in the face of challenging diseases.
### References: 1. Sánchez-Alba, L., et al. (2025). Structural basis for the human SENP5's SUMO isoform discrimination. *Nature Communications*. DOI: 10.1038/s41467-025-60029-4. 2. World Health Organization (2024). Post-translational modifications and disease: A global perspective. WHO Health Report.
Advertisement
Tags
Advertisement





